Independent
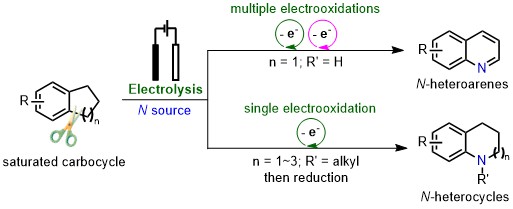
92.“Divergent synthesis of N-heterocycles from carbocycles enabled by electrochemical nitrogen atom insertion," Sun, G.-Q.; Wang, X.; Hu, R.; Rao, W.; Zhao, Y.;* Koh, M. J.* Nat. Synth. 2025, just accepted.
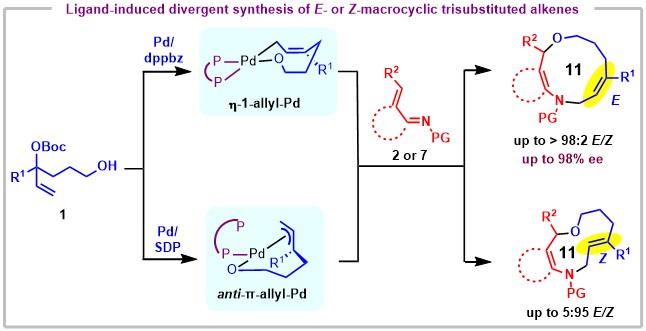
91.“Divergent access to E- or Z-trisubstituted medium-sized cycloalkenes by Pd-catalyzed cycloaddition," Zou, G.-F.; Lin, W.; Shi, L.; Yang, B.-M.;* Lan, Y.;* Zhao, Y.* Nat. Chem. 2025, in press.
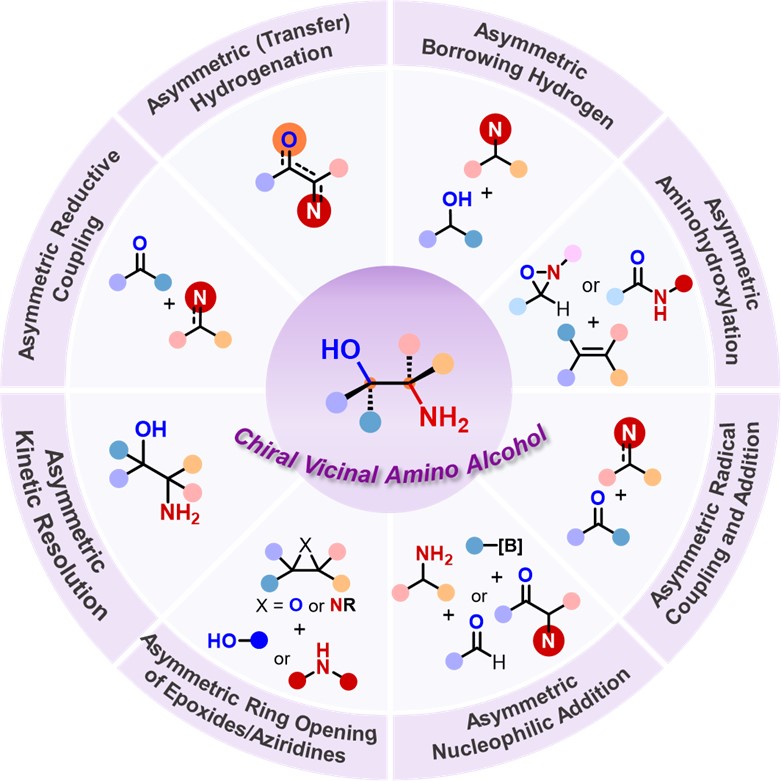
90.“Recent Advances in Catalytic Enantioselective Synthesis of Vicinal Amino Alcohols," Yan, X.; Fang, W.; Ng, J. X.; Li, J.; Liu, Y.; Zhang, B.;* Shao, P.-L.;* Zhao, Y.* Chem. Soc. Rev. 2025, 54, 7966-8018.
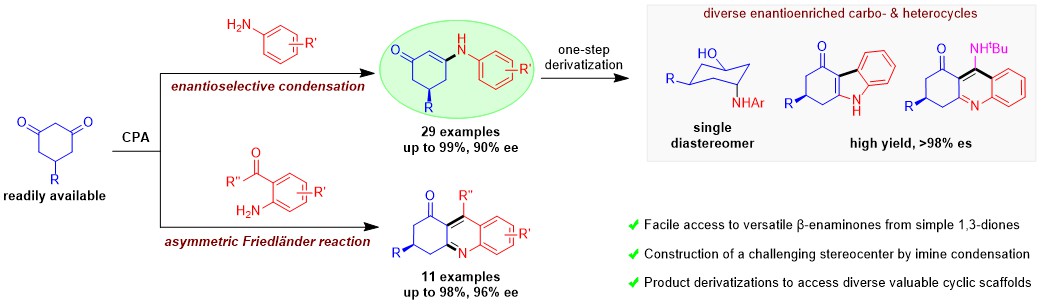
89.“Access to Chiral Cyclic β-Enaminones via Enantioselective Imine Condensation," Gao, Y.; Lin, Z.-K.; Liu, M.; Deng, J.; Zhang, T.; Yang, B.-M.; * Zhao, Y.* Angew. Chem. Int. Ed. 2025, 64, e202512405 (G.Y. and L.Z.K. contributed equally).
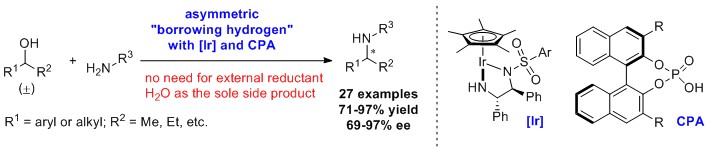
88.“Borrowing Hydrogen/Chiral Enamine Relay Catalysis Enables Di-astereo- and Enantioselective β-C-H Functionalization of Alcohols," Liaw, M. W.; Hirata, H.; Zou, G.-F.;* Wu, J.;* Zhao, Y.* J. Am. Chem. Soc. 2025, 147, 7721–7728.

87.“Desymmetrization of 1,4-Diynes by Allylic Alcohol-Triggered Redox Enyne Cycloisomerization," Wang, F.; Lin, W.; Yeh, C. H.; Lan, Y.;* Zhao, Y.* Angew. Chem. Int. Ed. 2025, 64, e202421153.

86.“Iridium-Catalyzed Enantioconvergent Construction of Piperidines and Tetrahydroisoquinolines from Racemic 1,5-Diols," Diao, H.; Liu, K.; Yu, R.; Chen, J.; Liu, Y.;* Yang, B.-M.;* Zhao, Y.* J. Am. Chem. Soc. 2025, 147, 610-618.
85.“Dynamic Asymmetric Diamination of Allylic Alcohols through Borrowing Hydrogen Catalysis: Diastereo-divergent Synthesis of Tetrahydrobenzodiazepines," Liu, Y.; Ji, P.; Zou, G.; Liu, Y.; Yang, B.-M.;* Zhao, Y.* Angew. Chem. Int. Ed. 2024, 63, e202410351.
84.“Asymmetric Amination of Primary Alcohols via Dynamic Kinetic Resolution: Enantioconvergent Access to Chiral Benzomorpholines," Gao, Y.; Hong, G.; Zhang, L.; Ye, K.-Y.; Cheng, J.; Yang, B.-M.;* Zhao, Y.;* CCS Chem. 2025, 7, 80-90 (G.Y. and H.G. contributed equally).
83. “Modular alkene synthesis from carboxylic acids, alcohols, and alkanes via integrated photocatalysis," Zeng, H.; Yin, R.; Zhao, Y.;* Ma, J.;* Wu, J.* Nat. Chem. 2024, 16, 1822-1830.
82.“Enantioselective Construction of Eight-Membered N-Heterocycles from Simple 1,3-Dienes via Pd(0) Lewis Base Catalysis," Pan, J.; Ho, T. O.; Chen, Y. C.;* Yang, B.-M.;* Zhao, Y.* Angew. Chem. Int. Ed. 2024, 63, e202317703.
81.“Scalable Synthesis of Antihistamines and Sensipar via Intensified Hydrogen Borrowing Methodology," Ng, X. Q.; Zhao, Y.;* Isoni, V.* ACS Sustain. Chem. Eng. 2023, 11, 12389-12396.
80. Invited review: “Enantioconvergent transformations of secondary alcohols through borrowing hydrogen catalysis," Gao, Y; Hong, G.; Yang, B.-M.;* Zhao, Y.* Chem. Soc. Rev. 2023, 52, 5541-5562.
79. Invited highlight: “When Remote C-H Activation Meets Planar Chirality," Yang, G.; Zhao, Y.* Sci. Bull. 2023, 68, 1595-1597.
78.“Concise Synthesis of Chiral Tricyclic Lactams by Tandem Dynamic Kinetic Asymmetric Reductive Amination/Lactamization Using Ammonium Salts," Wang, J.; Shi, Y.; Wang, F.; Huang, F.; Bai, S.-T.;* Zhao, Y.;* Zhang, X.* Angew. Chem. Int. Ed. 2023, 62, e202303868.
77. "Chiral Acid-Catalyzed Atroposelective Indolization Enables Access to 1,1-Indole-Pyrroles and Bisindoles Bearing a Chiral N–N Axis," Wang, L.-Y.; Miao, J.; Zhao, Y.;* Yang, B.-M.* Org. Lett. 2023, 25, 1553-1557.
76.“Direct Access to Chiral Aliphatic Amines by Catalytic Enantioconvergent Redox-Neutral Amination of Alcohols," Ng, X. Q.; Lim, C. S.; Liaw, M. W.; Quach, T. T.; Yang, B.-M.; Isoni, V.; Wu, J.* Zhao, Y.* Nat. Synth. 2023, 2, 572-580 (N.X.Q. and L.C.S. contributed equally).
75.“Iridium-Catalyzed Enantioconvergent Borrowing Hydrogen Annulation of Racemic 1,4-Diols with Amines," Liu, Y.; Diao, H.; Hong, G.; Edward, J.; Zhang, T.; Yang, G.; Yang, B.-M.;* Zhao, Y.* J. Am. Chem. Soc. 2023, 145, 5007-5016 (L.Y. and D.H. contributed equally).
74.“Enantioselective Access to Triaryl-2-pyrones with Monoaxial or Contiguous C-C Diaxes via Oxidative NHC Catalysis," Zhang, S.-C.; Liu, S.; Wang X.; Wang ,S.-J.; Yang, H.; Li, L.; Yang, B.; Wong, M. W.; Zhao, Y.; Lu, S.* ACS Catal. 2023, 13, 2565–2575.
73.“Anion Effect in Enantioselective Oxidative NHC Catalysis: Highly Efficient Kinetic Resolution of Tertiary alcohols and Beyond," Hu, D.; Poh, S. B.; Liu, F.; Tu, Z.; Wang, X.; Lu, S.;* Zhao, Y.* Org. Chem. Front. 2023, 10, 416-421.
72.“Economical Access to Diverse Enantiopure Tetrahydropyridines and Piperidines Enabled by Catalytic Borrowing Hydrogen," Ng, T. W.; Tao, R.; See, W. W. L.; Poh, S. B.; Zhao, Y.* Angew. Chem. Int. Ed. 2023, 62, e202212528 (N.T.W. and T.R. contributed equally).
71. Invited article: “Co/Zn Bimetallic Catalysis Enables Enantioselective Alkynylation of Isatins and α-Ketoesters Using Terminal Alkynes," Huang, R.-Z.; Ma, Z.-C.; Huang, Y.;* Zhao, Y.* J. Org. Chem. 2023, 88, 7755-7763.
Part of Special Issue: Modern Enantioselective Catalysis in Organic Chemistry
70. Invited review: “Enantioselective synthesis of indoles through catalytic indolization," Yang, B.-M.;* Ng, X. Q.; Zhao, Y.* Chem Catal. 2022, 2, 3048-3076.
69.“A green access to supported cinchona alkaloid amide catalysts for heterogeneous enantioselective allylsilylation of aldehydes and process intensity evaluation in batch and flow," Ng, X. Q.; Kang, M. H.; Toh, R. W.; Isoni, V.;* Wu, J.;* Zhao, Y.* Green Synth. Catal. 2022, 3, 272-277.
68.“Atroposelective Synthesis of 1,1’-Bipyrroles Bearing a Chiral N-N Axis: Chiral Phosphoric Acid Catalysis with Lewis Acid Induced Enantiodivergence," Gao, Y.; Wang, L.-Y.; Zhang, T.; Yang, B.-M.;* Zhao, Y.* Angew. Chem. Int. Ed. 2022, 61, e202200371 (VIP).
67.“Enantioselective Cascade Annulation of α-Amino-ynones and Enals Enabled by Gold and Oxidative NHC Relay Catalysis," Jiang, J.; Wang, X.; Liu, S.; Zhang, S.; Yang, B.; Zhao, Y.; Lu, S.* Angew. Chem. Int. Ed. 2022, 61, e202115464.
66.“Experimental and Computational Studies on the Directing Ability of Chalcogenoethers in Palladium-Catalyzed Atroposelective C–H Olefination and Allylation," Liao, G.; Zhang, T.; Jin, L.; Wang B.-J.; Xu, C.-K.; Lan, Y.;* Zhao, Y.;* Shi, B.-F.* Angew. Chem. Int. Ed. 2022, 61, e202115221.
65.“Tandem Catalytic Indolization/Enantioconvergent Substitution of Alcohols by Borrowing Hydrogen to Access Tricyclic Indoles," Yang, G.; Pan, J.; Ke, Y.-M.; Liu, Y.;* Zhao, Y.* Angew. Chem. Int. Ed. 2021, 60, 20689-20694.
64.“Redox-Enabled Direct Stereoconvergent Heteroarylation of Simple Alcohols," Liu, Y.; Tao, R.; Lin, Z.-K.; Yang, G.;* Zhao, Y.* Nat. Commun. 2021, 12, 5035.
63.“Catalytic Diastereo- and Enantioconvergent Synthesis of Vicinal Diamines from Diols through Borrowing Hydrogen," Pan, H.-J.; Lin, Y.; Gao, T.; Lau, K. K.; Feng, W.; Yang, B.;* Zhao, Y.* Angew. Chem. Int. Ed. 2021, 60, 18599-18604 (H.J.P. and Y.L. contributed equally).
62. "Catalytic Atroposelective Dynamic Kinetic Resolution of Biaryl Lactones with Activated Isocyanides," Qian, L.; Tao, L.-F.; Wang, W.-T.; Jameel, E.; Luo, Z.-H.; Zhang, T.; Zhao, Y.; Liao, J.-Y.* Org. Lett. 2021, 23, 5086-5091.
61.“Stereoselective Access to Polyfunctionalized Nine-Membered Heterocycles by Sequential Gold and Palladium Catalysis," Yang, G.; Ke, Y.-M.; Zhao, Y.* Angew. Chem. Int. Ed. 2021, 60, 12775-12780.
60.“Access to 5,6-Spirocycles Bearing Three Contiguous Stereocenters via Pd-Catalyzed Stereoselective [4 + 2] Cycloaddition of Azadienes," Sheikh Ismail S. N. F.; Yang, B.;* Zhao, Y.* Org. Lett. 2021, 23, 2884-2889.
59.“Desymmetrization of 1,3-Diones by Catalytic Enantioselective Condensation with Hydrazine," Yang , B.; Dai, J.; Luo, Y.; Lau, K. K.; Lan, Y.;* Shao, Z.;* Zhao, Y.* J. Am. Chem. Soc. 2021, 143, 4179-4186 (Y.B. and D.J. contributed equally).
58.“Ligand Coordination- and Dissociation-Induced Divergent Allylic Alkylations Using Alkynes," Huang, Y.; Ma, C.; Liu, S.; Yang, L.-C.; Lan, Y.;* Zhao, Y.* Chem 2021, 7, 812-826 (H.Y., M.C. and L.S. contributed equally).
57.“Nickel-Catalyzed Site- and Stereoselectivereductive Alkylalkynylation of Alkynes," Jiang, Y.; Pan, J.; Yang, T.; Zhao, Y.;* Koh, M. J.* Chem 2021, 7, 993-1005.
56.“Transformation of Corn Lignin into Sun Cream Ingredients," See, J. Y.; Song, S.; Xiao, Y,; Pham, T. T.; Zhao, Y.; Lapkin, A.; Yan, N.* ChemSusChem, 2021, 14, 1586-1594.
55.“Access to Substituted Cyclobutenes by Tandem [3,3]-Sigmatropic Rearrangement/[2+2] Cycloaddition of Dipropargylphosphonates under Ag/Co Relay Catalysis," Ni, Q.; Song, X.; Png, C. W.; Zhang, Y.;* Zhao, Y.* Chem. Sci. 2020, 11, 12329-12335 (N.Q. and S.X. contributed equally).
54.“Isothiourea-Catalyzed Atroposelective N-Acylation of Sulfonamides," Ong, J. Y.; Ng, X. Q.; Lu, S.;* Zhao, Y.* Org. Lett. 2020, 22, 6447-6451 (O.J.Y. and N.X.Q. contributed equally).
53.“Dynamic Kinetic Asymmetric Amination of Alcohols Assisted by Microwave: Stereo-convergent Access to Tetralin- and Indane-Derived Chiral Amines," Rong, Z.-Q.; Yu, Z.; Weng, C.; Yang, L.-C.; Lu, S.; Lan, Y.;* Zhao, Y.* ACS Catal. 2020, 10, 9464-9475.
52.“Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions," Yang, L.-C.; Wang, Y.-N.; Liu, R.; Luo, Y.; Ng, X. Q.; Yang , B.; Rong, Z.-Q.; Lan, Y.;* Shao, Z.;* Zhao, Y.* Nat. Chem. 2020, 12, 860-268 (Y.L.C. and W.Y.N. contributed equally).
51.“Nickel-Catalyzed Allylmethylation of Alkynes Using Allylic Alcohols and AlMe3: A Facile Access to Skipped Dienes and Trienes," Li, W.;* Yu, S.; Li, J.; Zhao, Y.* Angew. Chem. Int. Ed. 2020, 59, 14404-14408.
50.“Room-Temperature Guerbet Reaction with Unprecedented Catalytic Efficiency and Enantioselectivity," Ng, T. W.; Liao, G.; Lau, K. K.; Pan, H.-J.; Zhao, Y.* Angew. Chem. Int. Ed. 2020, 59, 11384-11389 (N.T.W. and L.G. contributed equally).
49.“Diastereo- and Atroposelective Synthesis of Bridged Biaryls Bearing an Eight-Membered Lactone through an Organocatalytic Cascade," Lu, S.; Ong, J.-Y.; Poh, S. B.; Liew, X.; Seow, C. S. D.; Wong, M. W.;* Zhao, Y.* J. Am. Chem. Soc. 2019, 141, 17062-17067.
48. “NHC-Catalyzed Atroposelective Acylation of Phenols: Access to Enantiopure NOBIN Analogs by Desymmetrization,” Lu, S.; Poh, S. B.; Rong, Z.-Q.;* Zhao, Y.* Org. Lett. 2019, 21, 6169-6172.
47. “Stereoconvergent, Redox-Neutral Access to Tetrahydroquinoxa-lines by Relay Catalytic Epoxide Opening/Amination of Alcohol,” Xu, G.; Yang, G.; Wang, Y.; Shao, P.-L.; Yau, J. N. N.; Liu, B.; Zhao, Y.; Sun, Y.; Xie, X.; Wang, S.; Zhang, Y.;* Xia, L.;* Zhao, Y.* Angew. Chem. Int. Ed. 2019, 58, 14082-14088 (X.G. and Y.G. contributed equally).
46. “Practical Access to Axially Chiral Sulfonamides and Biaryl Amino Phenols via Organocatalytic Atroposelective N-Alkylation,” Lu, S.; Ng, S. V. H.; Lovato, K.; Ong, J.-Y.; Poh, S. B.; Ng, X. Q.; Kurti, L.;* Zhao, Y.* Nat. Commun. 2019, 10, 3061.
45.“B(C6F5)3-Catalyzed Redox-Neutral β-Alkylation of Tertiary Amines using p-Quinone Methides via Borrowing Hydrogen,” Li, R.; Chen, Y.; Jiang, K.; Wang, F.; Lu, C.; Nie, J.; Chen, Z.; Yang, G.;* Chen, Y.-C.; Zhao, Y.;* Ma, Chao.* Chem. Commun. 2019, 55, 1217-1220.
44.“Ag-Catalyzed Thiocyanofunctionalization of Terminal Alkynes to Access Alkynylthiocyanates and α-Thiocyanoketones," See, J. Y.; Zhao, Y.* Org. Lett. 2018, 20, 7433-7436.
43.“Rhodium-Catalyzed Enantioconvergent Isomerization of Homoallylic and Bishomoallylic Secondary Alcohols," Huang, R.-Z.; Lau, K. K.; Li, Z.; Liu, T.-L.;* Zhao, Y.* J. Am. Chem. Soc. 2018, 140, 14647-14654.
42.“Cu-Catalyzed [3 + 3] Cycloaddition of Isocyanoacetates with Aziridines and Stereoselective Access to α,γ-Diamino Acids," Kok, G. P. Y.; Yang, H.; Wong, M. W.;* Zhao, Y.* Org. Lett. 2018, 20, 5112-5115.
41.“Divergent, Enantioselective Synthesis of Pyrroles, 3H Pyrroles and Bicyclic Imidazolines by Ag- or P-Catalyzed [3 + 2] Cycloaddition of Allenoates with Activated Isocyanides," Kok, G. P. Y.; Shao, P.-L.;* Liao, J.-Y.; Sheikh Ismail, S. N. F.; Yao, W.; Lu, Y.;* Zhao, Y.* Chem. Eur. J. 2018, 24, 10513-10520.
40.“Pd-Titanium Relay Catalysis Enables Switch of Alkoxide-π-Allyl to Dienolate Reactivity for Spiro-Heterocycle Synthesis," Yang, L.-C.; Tan, Z. Y.; Rong, Z.-Q.; Liu, R.; Wang, Y.-N.; Zhao, Y.* Angew. Chem. Int. Ed. 2018, 57, 7860-7864.
39.“Direct Enantioselective α-Allylation of Unfunctionalized Cyclic Ketones with Alkynes via Pd-Amine Cooperative Catalysis," Lee, J. T. D.; Zhao, Y.* Chem. Eur. J. 2018, 24, 9520-9524.
38.“Catalytic and Enantioselective Direct a‐Alkylation of 3‐Aryl and 3‐Alkyl Oxindole Using Quinine‐Derived Urea Catalyst," Paderes, M. C.;* Siau, W. Y.; Rong, Z.-Q.; Zhao, Y.* Chem. Select 2018, 3, 6160-6164.
37.“Visible-Light-Driven Alkyne Hydro-/Carbocarboxylation Using CO2 via Iridium/Cobalt Dual Catalysis for Divergent Heterocycle Synthesis," Hou, J.; Ee, A.; Feng, W.; Xu, J.-H.; Zhao, Y.;* Wu, J.* J. Am. Chem. Soc. 2018, 140, 5257-5263.
36.“Transition Metal-Free Decarboxylative Propargylic Substitution/ Cascade Cyclization with Azolium Enolates or Acyl Anions," Lu, S.; Ong, J.-Y.; Poh, S. B.; Tsang, T.; Zhao, Y.* Angew. Chem. Int. Ed. 2018, 57, 5714-5719 (L.S. and O.J.-Y. contributed equally).
This article was featured in Synfacts.
35.“FeCl3-Catalyzed Dimerization/Elimination of 1,1-Diarylalkenes: Efficient Synthesis of Functionalized 4H-Chromenes," Ma, C;* Zhao, Y.* Org. Biomol. Chem. 2018, 16, 703-706.
34.“Highly Regio- and Stereodivergent Access to 1,2-Amino Alcohols or 1,4-Fluoro Alcohols by NHC-Catalyzed Ring Opening of Epoxy enals," Poh, S. B.; Ong, J.-Y.; Lu, S.;* Zhao, Y.* Angew. Chem. Int. Ed. 2018, 57, 1645-1649.
33.“Pd-Catalyzed Enantioselective [6+4] Cycloaddition of Vinyl Oxetanes with Azadienes to Access Ten-Membered Heterocycles," Wang, Y.-N.; Yang, L.- C.; Rong, Z.-Q.; Liu, T.-L.; Liu, R.; Zhao, Y.* Angew. Chem. Int. Ed. 2018, 57, 1596-1600 (W.Y.N. and Y.L.C. contributed equally).
This article was featured in Synfacts.
32.“Desymmetrizing Enantio- and Diastereoselective Selenoetherification through Supramolecular Catalysis," See, J. Y.; Yang, H.; Zhao, Y.; Wong, M. W.;* Ke, Z.;* Yeung, Y.-Y.* ACS Catal. 2018, 8, 850-858.
31.“Nickel-catalyzed Difunctionalization of Allyl Moieties Using Organoboronic Acids and Halides with Divergent Regioselectivities," Li, W.; Boon, J. K.; Zhao, Y.* Chem. Sci. 2018, 9, 600-607.
30.“Nine-Membered Benzofuran-Fused Heterocycles: Enantioselective Synthesis by Pd-Catalysis and Rearrangement via Transannular Bond Formation," Rong, Z.-Q.; Yang, L.-C.; Liu, S.; Yu, Z.; Wang, Y.-N.; Tan, Z. Y.; Huang, R.-Z.; Lan, Y.;* Zhao, Y.* J. Am. Chem. Soc. 2017, 139, 15304-15307 (R.Z.Q. and Y.L.C. contributed equally).
This article was featured in Synfacts.
29.“Catalyst-Enabled Scaffold Diversity: Highly Chemo- and Stereoselective Synthesis of Tricyclic Ketals and Triarylmethanes," Liao, J.-Y.; Ni, Q.; Zhao, Y.* Org. Lett. 2017, 19, 4074-4077.
This article was featured in Synfacts.
28.“Three-Component Reactions of Isocyanoacetates, Amines and 3-Formylchromones Initiated by an Unexpected aza-Michael Addition," Liao, J.-Y.; Yap, W. J.; Wu, J.; Wong, M. W.;* Zhao, Y.* Chem. Commun. 2017, 53, 9067-9070.
27.“Enantioselective Synthesis of Tetrahydroquinolines Using Borrowing Hydrogen: Cooperative Catalysis by Achiral Iridacycle and Chiral Phosphoric Acid," Lim, C. S.; Quach, T. T.; Zhao, Y.* Angew. Chem. Int. Ed. 2017, 56, 7176-7180 (VIP).
This article was featured in Synfacts.
26.“Divergent Reactivities in Fluoronation of Allylic Alcohols: Synthesis of Z-Fluoroalkenes via Carbon-Carbon Bond Cleavage," Liu, T.-L.; Wu, J.; Zhao, Y.* Chem. Sci. 2017, 8, 3885-3890.
25.“Rhodium-Catalyzed Enantioselective Isomerization of Secondary Allylic Alcohols," Liu, T.-L.; Ng, T. W.; Zhao, Y.* J. Am. Chem. Soc. 2017, 139, 3643-3646.
This article was featured in Synfacts.
24.“Construction of Nine-Membered Heterocycles through Palladium-Catalyzed Formal [5 + 4] Cycloaddition,” Yang, L.-C.; Rong, Z.-Q.; Wang, Y.-N.; Tan, Z. Y.; Wang, M. Zhao, Y.* Angew. Chem. Int. Ed. 2017, 56, 2927-2931 (Y.L.C. and R.Z.Q. contributed equally).
This article was featured in Synfacts.
23.“Access to Enantiopure Triarylmethanes and 1,1-Diarylalkanes by NHC-Catalyzed Acylative Desymmetrization,” Lu, S.; Song, X.; Poh, S. B.; Yang, H.; Wong, M. W.;* Zhao, Y.* Chem. Eur. J. 2017, 23, 2275-2281(L.S. and S.X. contributed equally).
22.“Acid-Assisted Ru-Catalyzed Enantioselective Amination of 1,2-Diols through Borrowing Hydrogen,” Yang, L.-C.; Wang, Y.-N.; Zhang, Y.;* Zhao, Y.* ACS Catalysis, 2017, 7, 93-97.
This article was featured in Synfacts.
21.“Formal [3 + 2] cycloaddition of α-unsubstituted isocyanoacetates and methyleneindolinones: enantioselective synthesis of spirooxindoles,” Peng, X.-J.; Ho, Y. A.; Wang, Z.-P.; Shao, P.-L.;* Zhao, Y.;* He, Y.* Org. Chem. Front. 2017, 4, 81-85.
20.“Access to Acyclic (Z)-Enediynes via Alkyne Trimerization: Cooperative Bimetallic Catalysis Using Air as the Oxidant,” Lee, J. T. D.; Zhao, Y.* Angew. Chem. Int. Ed. 2016, 55, 13872-13876.
19.“Stereoselective 1,6-Conjugate Addition/Annulation of Para-Quinone Methides with Vinyl Epoxides/Cyclopropanes,” Ma, C; Huang, Y.; Zhao, Y.* ACS Catalysis, 2016, 6, 6408-6412 (M.C. and H.Y. contributed equally).
This article was featured in Synfacts.
18.“Asymmetric Transfer Hydrogenation of Imines using Alcohol: Efficiency and Selectivity Are Affected by the Hydrogen Donor,” Pan, H.-J.; Zhang, Y.; Shan, C.; Yu, Z.; Lan, Y.*; Zhao, Y.* Angew. Chem. Int. Ed. 2016, 55, 9615-9619.
17. "Cobalt-Catalyzed Enantioselective Vinylation of Activated Ketones and Imines," Huang, Y.; Huang, R.-Z.; Zhao, Y.* J. Am. Chem. Soc. 2016, 138, 6571-6576 (H. Y. and H. R.-Z. contributed equally).
16. "Catalyst-Enabled Diastereodivergent aza-Diels-Alder Reaction: Complementarity of N-Heterocyclic Carbene and Chiral Amine," Rong, Z. Q.; Wang, M.; Chow, C. H. E.; Zhao, Y.* Chem. Eur. J. 2016, 22, 9483-9487 (R.Z.Q. and W.M. contributed equally).
15.“Iron-catalyzed transfer hydrogenation of imines assisted by an iron-based Lewis acid,” Pan, H.-J.; Ng, T. W.; Zhao, Y.* Org. Biomol. Chem. 2016, 14, 5490-5493 (Invited article for “New Talent Issue”).
14.“Cobalt-Catalyzed Allylation of Heterobicyclic Alkenes: Ligand-Induced Divergent Reactivities,” Huang, Y.; Ma, C.; Lee, Y. X.; Huang, R.-Z.; Zhao, Y.* Angew. Chem. Int. Ed. 2015, 54, 13696-13700.
This article was featured in Synfacts.
13.“Iron-catalyzed amination of alcohols assisted by Lewis acid,” Pan, H.-J.; Ng, T. W.; Zhao, Y.* Chem. Comm. 2015, 51, 11907–11910.
12. "Phase-Transfer-Catalyzed Enantioselective α-Hydroxylation of Acyclic and Cyclic Ketones with Oxygen," Sim, S. B. D.; Wang, M.; Zhao, Y.* ACS Catal. 2015, 5, 3609-3612.
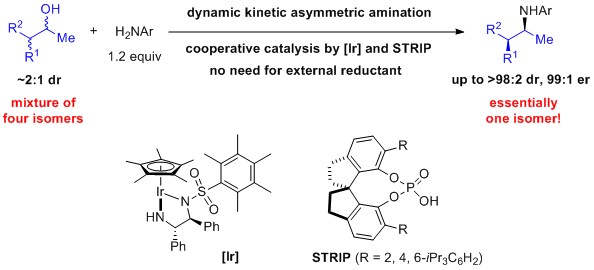
11. "Dynamic Kinetic Asymmetric Amination of Alcohols: From A Mixture of Four Isomers to Diastereo- and Enantiopure a-Branched Amines," Rong, Z. Q.; Zhang, Y.; Chua, R. H. B.; Pan, H.-J.; Zhao, Y.* J. Am. Chem. Soc. 2015, 137, 4944-4947 (R.Z.Q. and Z.Y. contributed equally).
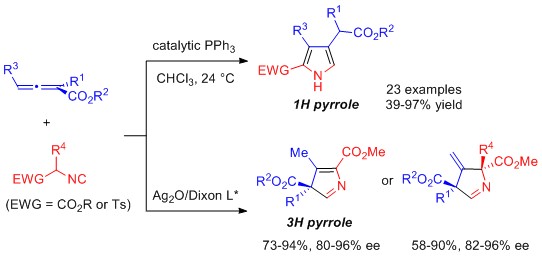
10. "Catalytic Divergent Synthesis of 3H or 1H Pyrroles by [3+2] Cyclization of Allenoates with Activated Isocyanides," Liao, J.-Y.; Shao, P.-L.; Zhao, Y.* J. Am. Chem. Soc. 2015, 137, 628-631 (L.J.Y. and S.P.L. contributed equally).
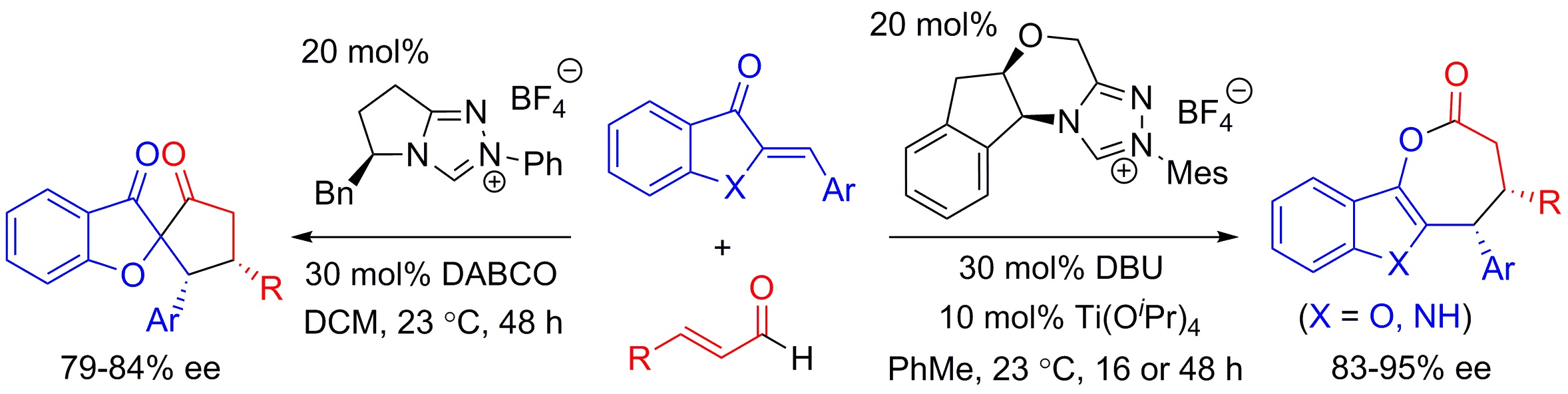
9. "Stereoselective Synthesis of e-Lactones or Spiro-Heterocycles through NHC-Catalyzed Annulation: Divergent Reactivity by Catalyst Control," Wang, M.; Rong, Z.-Q.; Zhao, Y.* Chem. Comm. 2014, 50, 15309-15312 (W.M. and R.Z.Q. contributed equally).
8. "Kinetic Resolution of 1,1’-Biaryl-2,2’-Diols and Amino Alcohols through NHC-Catalyzed Atroposelective Acylation," Lu, S.; Poh, S. B.; Zhao, Y.* Angew. Chem. Int. Ed. 2014, 53, 11041-11045.
This article was featured in Synfacts.
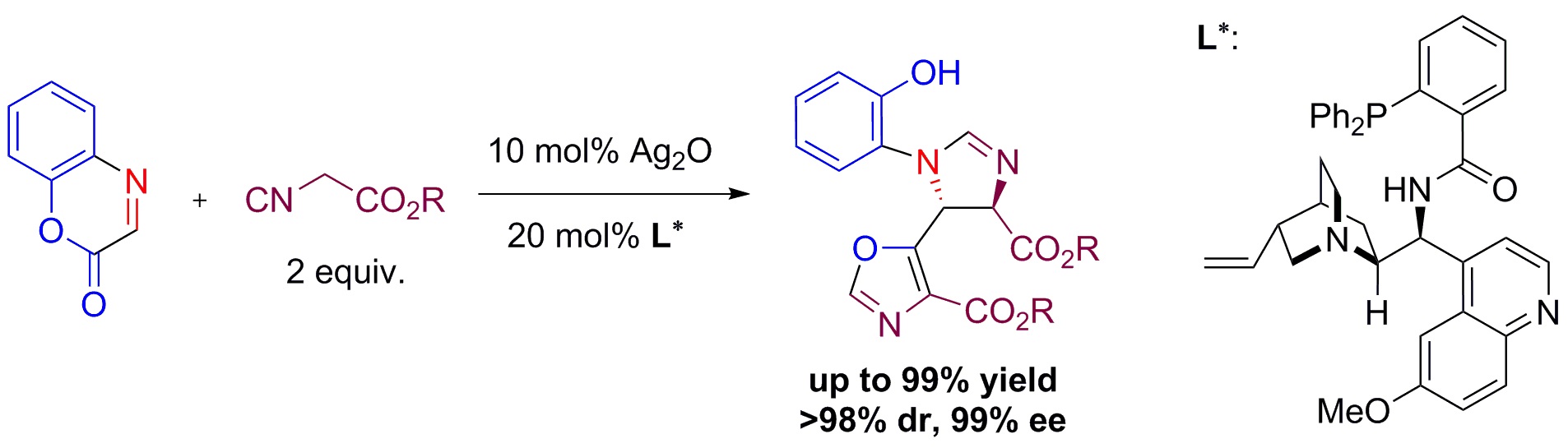
7. "Highly Diastereo- and Enantioselective Ag-Catalyzed Double [3+2] Cyclization of a-Imino Esters with Isocyanoacetate," Shao, P.-L.; Liao, J.-Y.; Ho, Y. A.; Zhao, Y.* Angew. Chem. Int. Ed. 2014, 53, 5435-5439.
This article was featured in Synfacts.
6. "Enantioselective Oxidation of 1,2-Diols with Quinine-derived Urea Organocatalyst," Rong, Z.-Q.; Pan, H.-J.; Yan, H.-L.; Zhao, Y.* Org. Lett. 2014, 16, 208−211.
5. "Catalytic Enantioselective Amination of Alcohols by the Use of Borrowing Hydrogen Methodology: Cooperative Catalysis by Iridium and a Chiral Phosphoric Acid," Zhang, Y.; Lim, C.-S.; Sim, D. S. B.; Pan, H.-J.; Zhao, Y.* Angew. Chem. Int. Ed. 2014, 53, 1399-1403.
This article was featured in Synfacts and highlighted in ChemSusChem.
4. "Practical, Highly Stereoselective Allyl- and Crotylsilylation of Aldehydes Catalyzed by Readily Available Cinchona Alkaloid Amide," Huang, Y.; Yang, L.; Shao, P.; Zhao, Y.* Chem. Sci. 2013, 4, 3275-3281.
3. Invited highlight: "Kinetic Resolution of 3-Hydroxy-3-Substituted Oxindoles through NHC-Catalyzed Oxidative Esterification," Lu, S.; Poh, S. B.; Siau, W.-Y.; Zhao, Y.* Synlett, 2013, 24, 1165-1169.
2. “Kinetic Resolution of Tertiary Alcohols: Highly Enantioselective Access to 3-Hydroxy-3-Substituted Oxindoles,” Lu, S.; Poh, S. B.; Siau, W.-Y.; Zhao, Y.* Angew. Chem. Int. Ed. 2013, 52, 1731-1734.
This article was featured in Synfacts.
1. Invited review: “Stereoselective Synthesis of Z-Alkenes,” Siau, W.-Y.; Zhang, Y.; Zhao, Y.* Top. Curr. Chem. 2012, 327, 33-58.
Previous
Publications
7. “Preparation of Highly Pure Disubstituted E Olefins through Mo-Catalyzed Z-Selective Ethenolysis of Stereoisomeric Mixtures,” Marinescu, S. C.; Levine, D. S.; Zhao, Y.; Schrock, R. R.;* Hoveyda, A. H.* J. Am. Chem. Soc. 2011, 133, 11512-11514.
6. “Regiodivergent Reactions through Catalytic Enantioselective Silylation of Chiral Diols. Synthesis of Sapinofuranone A,” Rodrigo, J.; Zhao, Y.; Hoveyda, A. H.;* Snapper, M. L.* Org. Lett. 2011, 13, 3778-3781.
5. “Endo-Selective Enyne Ring-Closing Metathesis Promoted by Stereogenic-at-W Mono-Pyrrolide Complexes,” Zhao, Y.; Hoveyda, A. H.;* Schrock, R. R.* Org. Lett. 2011, 13, 784-787.
4. “Highly Z-Selective Metathesis Homocoupling of Terminal Olefins,” Jiang, A. J.; Zhao, Y.; Hoveyda, A. H.;* Schrock, R. R.* J. Am. Chem. Soc. 2009, 131, 16630-16631.
3. “Kinetic Resolution of 1,2-Diols through Highly Site- and Enantioselective Catalytic Silylation,” Zhao, Y.; Mitra, A. W.; Hoveyda, A. H.;* Snapper, M. L.* Angew. Chem. Int. Ed. 2007, 44, 8471-8474.
2. “Enantioselective Silyl Protection of Alcohols Catalysed by an Amino-Acid-Based Small Molecule,” Zhao, Y.; Rodrigo, J.; Hoveyda, A. H.;* Snapper, M. L.* Nature 2006, 443, 67-70.
1. “Proline-Based N-Oxides as Readily Available and Modular Chiral Catalysts. Enantioselective Reactions of Allyltrichlorosilane with Aldehydes,” Traverse, J. F.; Zhao, Y.; Hoveyda, A. H.;* Snapper, M. L.* Org. Lett. 2005, 7, 3151-3154.
Patents
2. “Highly Z-Selective Olefin Metathesis,” Schrock, R. R.; Hoveyda, A. H.; Jiang, A. J.; Zhao, Y.; Flook, M. M. Publication 2011, # US-2011-0077421-A1.
1. “Catalytic Enantioselective Silylations of Substrates,” Snapper M. L.; Hoveyda A. H.; Rodrigo, J.; Zhao, Y. PCT Int. Appl. 2007, # WO2007082026.